| Project Manager | Dr. Jakob Steuer |
|---|---|
| Principal Investigator | Prof. Dr. Christine Peter |
| Affiliation | University of Konstanz |
| Project duration | bwHPC-S5 Phase II-III |
| Platform used | bwForCluster MLS&WISO, bwForCluster Helix |
| bwHPC Domain | Soft Matter |
| DOI of Publication | 10.1093/nargab/lqae132 |
| Project added | 20.02.2025 |
Riboswitches are RNA-based regulatory elements that are improtant in bacterial gene expression. They act by changing their structure in response to small molecules called ligands. These dynamic structural changes, known as 'switching', enable the riboswitch to modulate the expression of genes, highlighting its critical role in cellular regulation.
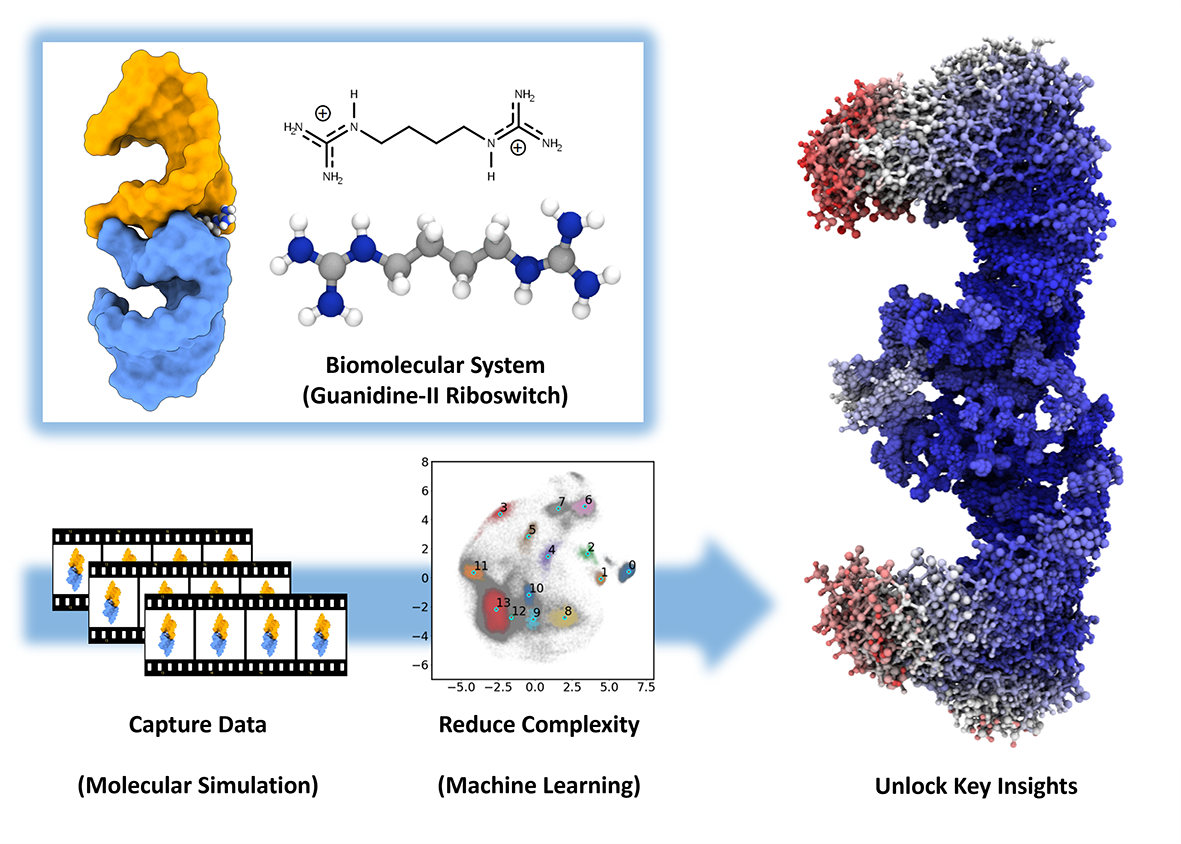
This study combines experimental investigations with extensive molecular dynamics simulations and machine learning techniques to explore the intricacies of the guanidine-II riboswitch. It is part of a family of riboswitches that sense guanidine, potentially enabling bacteria to utilize guanidine as a nitrogen source. A deeper understanding of their function and the underlying mechanisms not only provides valuable insights into fundamental microbial metabolism and gene regulation, but also offers promising applications in fields such as synthetic biology and pharmaceuticals, where controlling gene expression is key. By using specifically designed bivalent ligands, the study explored how these molecules bind to the riboswitch, impact the molecular interactions and trigger conformational changes of the RNA. Thus, they can play an important role for unravelling the detailed mechanisms of ligand-induced switching.
Simulating these structural transitions and binding events at the atomic scale poses significant computational challenges, requiring vast amounts of processing power. The bwHPC project provided the essential computational resources to manage these intensive simulations, and enabled the efficient analysis of large datasets through machine learning. This integration of computational power and data analysis was instrumental in discovering key aspects of ligand-riboswitch interactions.
In the molecular simulations, spontaneous ligand binding events were observed and a previously unknown, ligand-dependent base-stacking interaction in the guanidine-II riboswitch was identified. The results demonstrate that for this system, the bivalent ligands can significantly increase binding affinity compared to natural ligands. In vivo experiments confirmed these findings by highlighting cooperative binding effects.
This study paves the way for the development of high-affinity ligands that can modulate gene expression and highlights the importance of exploring ligand-dependent interactions in riboswitches. Future efforts will focus on further refining our understanding of the relationship between ligand binding and structural dynamics, potentially leading to new biotechnological tools and therapeutic strategies for controlling bacterial gene expression.